Abstract
Background: Treatment recommendations for polycythemia vera (PV) are age-based and reserve cytoreductive agents for patients (pts) older than 60, with some exceptions such as thrombosis history (Marchetti M, et al. Lancet Haematol 2022; Ali H, et al. NCCN Guidelines Version 2.2022). Effective, potentially life-prolonging cytoreductive treatment such as interferon-alpha (IFN) and hydroxyurea (HU) is often deferred in younger pts due to a presumption that treatment toxicity exceeds its potential benefit (Abu-Zeinah G, et al. Leuk 2021). In this systematic review, we examine the safety of cytoreductive agents in PV pts younger than 60, and discuss the implications of our findings to the clinical care of younger PV pts.
Methods: We used the databases PubMed, Scopus, Web of Science and Embase to identify relevant articles with search terms "polycythemia vera" and one of: hydroxyurea, hydroxycarbamide, interferon, ruxolitinib, cytoreductive. Inclusion criteria were predefined as: prospective studies including clinical trials, and retrospective studies including case series with at least 20 pts; pts with a diagnosis of PV; median age 18-60 years (yr) at enrollment; and the use of IFN, HU, or ruxolitinib (RUX). Data on treatment discontinuation for toxicity (DCT), frequency of grade 3-4 adverse events (AEs), and duration of follow-up were collected. Toxicity data were pooled using random-effects models with weights assigned based on study size and duration (patient-years). The derived annual DCT rate assumes linear correlation of toxicity with treatment time, and the pooled analysis assumes Poisson distribution of DCT probability.
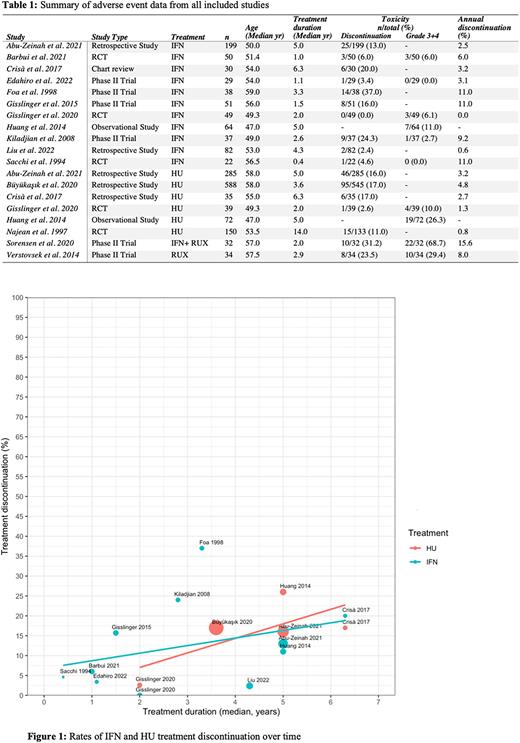
Results: The search resulted in 691 articles. Titles and abstracts were independently screened by two reviewers with 91% concordance and 102 articles were ultimately selected for full-text review. Of those, 18 met inclusion criteria and were selected for data extraction. JBI quality scores were calculated and ranged between 0.6 and 1. The median age of PV patients in these studies was between 47 and 59 yr. On IFN (n= 760, 12 studies), the reported DCT ranged from 4.6-37% over median durations of 0.4-6.3 yr (Table 1). The pooled DCT was 13% (95% CI 2.7-23%) and annual DCT rate for IFN was 3.2% (95% CI 0-6.4%). Of note, there was significant heterogeneity in reported DCT between studies (Cochran's Q = 29, p<0.001, and I2 = 70%), perhaps related to differences in dosing. Commonly reported causes for IFN DCT include fatigue, flu-like symptoms, and pneumonia. On HU (n= 1169, 6 studies), the reported DCT ranged from 2.6-17% over median durations of 2-14 yr (Table 1). The pooled DCT for HU was 15% (95% CI 6.9-24%) and annual DCT rate was 3% (95% CI 1-5%). There was no significant heterogeneity in reported HU DCT between studies (Q = 6.4, p=0.3, and I2 = 27%). Commonly reported causes for HU DCT included gastrointestinal symptoms and skin ulcers. Only one study was available for RUX and reported a DCT of 23.5% over 2.9 yr.
Discussion: PV-related complications and mortality is higher than expected in younger pts (Abu-Zeinah K, et al. Leuk Lymphoma 2022; Abu-Zeinah G, et al. Leuk 2021), but cytoreductive treatment is often deferred - until patients are older or experience symptoms, thrombosis, or progression - because of toxicity concerns. In this systematic review focused on younger PV cohorts, we found the annual rate of DCT with IFN or HU to be relatively low at 3-3.2%. Additional long-term data is required because most patients in these studies were treated for less than 10 yr. Nevertheless, given the excess risks of thrombosis, progression and death in PV patients compared to age-matched controls; and the potential for IFN or HU to safely reduce those risks, we continue to advocate for earlier intervention in young PV pts. Treatment recommendations should account for these findings, particularly as PV is being diagnosed more frequently at a younger age.
Acknowledgements: Mr. Sa'ad Laws for library and systematic review support.
Disclosures
Kucine:Protagonist Therapeutics: Other: Member, Safety monitoring committee. Scandura:CR&T: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Research Funding; Sumitomo Pharma Oncology, Inc: Consultancy; European Leukemia net: Honoraria, Other: Travel fees; MPN-RF: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal